
Compounding for Clinical Trials
Partner With Maida Pharmacy
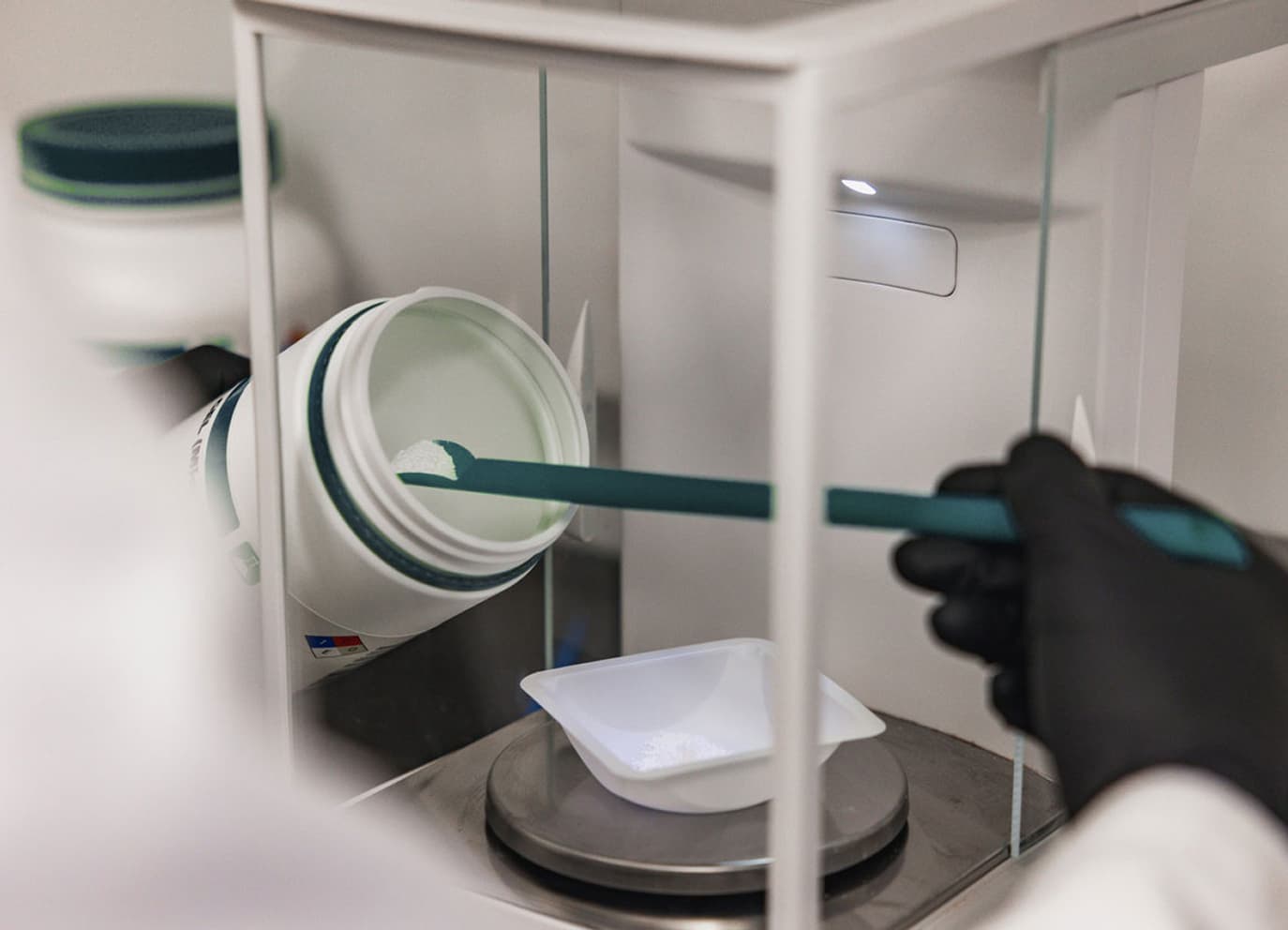
Maida Pharmacy provides precise, compliant, and flexible compounding services to support pharmaceutical and academic research.
Quality, Compliance, and Expertise
Where Science Meets Precision
We operate under strict USP compliance to ensure every formulation meets research and safety standards. Our internal QA/QC team continuously reviews, tests, and documents every process to maintain accuracy and consistency.
503A Compounding
We operate under strict USP compliance to ensure every formulation meets research and safety standards.
QA/QC Program
Our internal QA/QC team continuously reviews, tests, and documents every process to maintain accuracy and consistency.
Regulatory Expertise
We work closely with regulatory frameworks and guidance to ensure your trial materials meet all applicable requirements.

Flexible Solutions for Research Teams
Formulation Without Limitations
We support early exploratory trials by compounding microdoses, validating stability, and optimizing delivery parameters for Phase 0, 1, and 2 trials.
Phase Zero
We support early exploratory trials by compounding microdoses, validating stability, and optimizing delivery parameters.
Phase One
We compound small-scale formulations for human safety trials, enabling dose escalation studies with tight tolerances.
Phase Two
We produce intermediate-scale batches to test efficacy and refine dosing in patient cohorts under blinded conditions.
Dosage Form Versatility
- Oral Medications Including Capsules, Suspensions, and Solutions
- Modified Release Formulations
- Topical Delivery Systems - Creams and Gels
- Alternative Bases for Improved Drug Delivery
- Nasal Sprays
Personalization
- Small Batch
- Blinding or Unblinding
- Unique Strengths of Active Pharmaceutical Ingredients
Efficient, Compliant, and Cost-Effective
The Maida Advantage
503A Model of Compounding
- USP Chapter compliance instead of cGMP
- Lower project costs
- Access to critical formulations
Quality Assurance & Control
- Internal QA/QC team oversight
- Regulatory collaboration
Logistics & Operations
- 90+ years of compounding experience
- Comprehensive product transport, storage, and distribution
